Shapes and Patterns in Nature
There are so many colors, shapes, and patterns in nature.
- Seashells
- Animal Skins (Zebra, Leopard)
- Butterflies
- Shape of Plants
- Flowers (Sun Flower)
- Fruits (Pineapple)
How do we explain these from perspective of science? There are several branches of science which have explored these questions for decades. There are Reaction Diffusion Models and Cellular Automata models explaining development of patterns on seashells, plants and animal skins. There is L-system developed by Aristid Lindenmayer to explain development of plants. It is a fascinating subject.
From Exploring Complex Forms in Nature Through Mathematical Modeling: A Case on Turritella Terebra
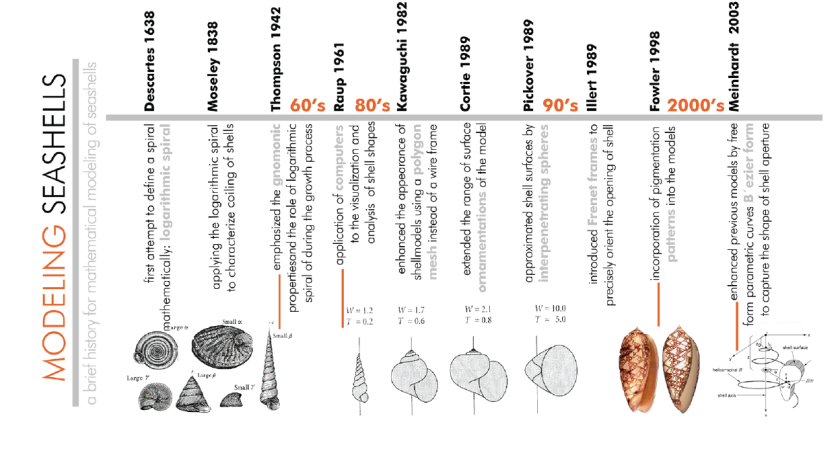
There are several studies have been carried out in a number of scientific disciplines, such as mathematics, biology, paleontology and computer engineering to understand and decipher the relations of the seashells complex forms. Starting with Descartes, Figure 4 shows a time line in which many investigators having focused on the curves of these shells and their mathematical properties. They all outlined a number of mathematical relations that control the overall geometry of seashells.
After examining the existing seashell models in literature it is seen that they all followed Raup’s model which roughly abstracts the seashell form using three parameters; whorl (rate of expansion of the generating curve), distance (relative distance between the generating curve and axis of coiling), and translation (the change of the cone’s movement along an axis with respect to the whorl), an ellipse as the whorl cross-section as well. However, it is clear from the observations of actual shells (Figure 5) that the cross-section is more complex than the input that the three parameters allow. In the pursuit of realistic visualizations, Kawaguchi enhanced the appearance of shell models using filled polygons which represented the surface of shells more convincingly than line drawings. Similar techniques were used subsequently by Oppenheimer (1986). A different approach was adopted by Pickover (1989) who approximated shell surfaces by using interpenetrating spheres. Illert (1989) introduced Frenet Frames (Bronsvoort, 1985) to precisely orient the opening of a shell. His model also captured a form of surface sculpture. Cortie (1989) studied the pattern forms on the surface of the shell model (Meinhardt, 2003). Finally, the model of seashell geometry by Fowler et al. (2003) was similar to that introduced by Raup, and was the first to implement free-form cross sections using a Bézier curve (Farin, 2002 Rogers, 2001) as the input. It can be claimed that, studies above all focused on modeling the appearance of the shell surface.
All these approaches can be considered as a milestone for their era, as each model reflects the observation and tools of measurement, modeling and technologies of their time. In all these approaches seashells were modeled as a single surface, as a twodimensional object, and embedded in three-dimensional space. Today, such modeling research should be carried out employing observation tools, knowledge, information, and computational technologies to the maximum extent. For this reason, we developed a mathematical model that can be transformed into a computational model for further studies (such as overall behavior of shells, form-structure relations, form finding explorations etc.) to explore potentials of such optimized forms.
From Exploring Complex Forms in Nature Through Mathematical Modeling: A Case on Turritella Terebra
From Computational models of plant development and form
A broad program of using mathematical reasoning in the study of the development and form of living organisms was initiated almost 100 yr ago by D’Arcy Thompson (1942) in his landmark book On Growth and Form (see Keller, 2002, for a historical analysis). One of his most influential contributions was the ‘theory of transformations’, which showed how forms of different species could be geometrically related to each other. The theory of transformations was extended to relate younger and older forms of a developing organism (Richards & Kavanagh, 1945), but did not incorporate the formation and differentiation of new organs. This limitation was addressed a quarter of a century later by Lindenmayer (1968, 1971), who introduced an original mathematical formalism, subsequently called L-systems, to describe the development of linear and branching structures at the cellular level. By the mid 1970s, computational models based on Lsystems and other formalisms had been applied to study several aspects of plant development, including the development of leaves and inflorescences, and the formation of phyllotactic patterns (Lindenmayer, 1978). The questions being asked included the impact of distinct modes of information transfer (lineage vs interaction) on plant development, and the relationship between local development and global form. Similar interests underlied the independent pioneering work of Honda and co-workers on the modeling of trees (Honda, 1971; Borchert & Honda, 1984).
Another class of models was pioneered by Turing (1952), who showed mathematically that, in a system of two or more diffusing reagents, a pattern of high and low concentrations may spontaneously emerge from an initially uniform distribution. This was a surprising result, as it appeared to contradict the second law of thermodynamics: the general tendency of systems to proceed from more organized states toward disorder (the apparent paradox is resolved by jointly considering the reaction–diffusion system and its surroundings). Related models were introduced, under the name of activator–inhibitor and activator-substrate (depletion) systems, by Gierer & Meinhardt (1972), and extensively investigated by Meinhardt (1982). Reaction–diffusion systems showed how, in principle, molecular-level interactions may lead to morphogenesis and differentiation. In plants, reaction– diffusion-type models have been used to explain the patterning of trichomes in leaves and hair cells in roots (Digiuni et al., 2008; Savage et al., 2008; Jo¨nsson & Krupinski, 2010; Benı´tez et al., 2011). Nevertheless, the extent to which reaction–diffusion models apply to the plant kingdom appears to be limited (Kepinski & Leyser, 2005; Berleth et al., 2007). A significant role is played instead by mechanisms involving active transport of the plant hormone auxin (Section V). In some cases, such as the generation of phyllotactic patterns, this reliance on active transport is difficult to explain in evolutionary terms, as reaction–diffusion systems can generate the same patterns. Spatio-temporal coordination of other developmental processes, however, such as bud activation, requires long-distance signaling. Active transport may thus have evolved to overcome the limitations of diffusion, which is very slow over long distances (Crick, 1971).
In the last decade, computational modeling has become a mainstream technique in developmental plant biology, as reflected in numerous reviews (e.g. Prusinkiewicz, 2004b; Prusinkiewicz & Rolland-Lagan, 2006; Grieneisen & Scheres, 2009; Chickarmane et al., 2010; Jo¨nsson&Krupinski, 2010; Jo¨nsson et al., 2012). On the one hand, the sequencing of the human genome put in focus the chasm between knowing the genome of an organism and understanding how this organismdevelops and functions.Computational models bridge this chasm. On the other hand, successes of early conceptual models that relate patterns of gene expression to the form of animals (Lawrence, 1992) and plants (Coen & Meyerowitz, 1991) have prompted a quest for a comprehensive, mechanistic understanding of development (Coen, 1999). Current experimental techniques for tracking growth and observing marked proteins in living tissues (Reddy et al., 2004; Fernandez et al., 2010) are yielding a wealth of data that correlate molecular-level processes with plant development and form. Computational models play an increasingly important role in interpreting these data.
The use of models has been accelerated by the advancements in computer hardware, software, and modeling methodologies. General-purpose mathematical software (e.g. Mathematica and MATLAB), modeling programs built on the basis of this software (e.g. GFtbox, Kennaway et al., 2011) and specialized packages for modeling plants (e.g. the Virtual Laboratory and L-studio (Prusinkiewicz, 2004a), OpenAlea (Pradal et al., 2008) and VirtualLeaf (Merks et al., 2011)) facilitate model construction, compared with general-purpose programming languages. Furthermore, current computers are sufficiently fast to simulate and visualize many models at interactive or close-to-interactive rates, which is convenient for model exploration.
From The reaction-diffusion system: a mechanism for autonomous pattern formation in the animal skin
In his paper entitled ‘The chemical basis of morphogenesis’ Turing presented a ground-breaking idea that a combination of reaction and diffusion can generate spatial patterns (Turing 1952). In the paper, he studied the behaviour of a complex system in which two substances interact with each other and diffuse at different diffusion rates, which is known as the reaction–diffusion (RD) system. Turing proved mathematically that such system is able to form some characteristic spatio-temporal patterns in the field. One of the most significant deviations is s formation of a stable periodic pattern. He stated that the spatial pattern generated by the system might provide positional information for a developing embryo.
In spite of the importance of the idea in the developmental biology, his model was not accepted by most experimental biologists mainly because there were no experimental technologies available to test it. Therefore, most of those who took over and developed the Turing’s idea were applied mathematicians and physicists. They proposed various types of model that developed Turing’s original equation to fit real, naturally occurring phenomena (Meinhardt 1982; Murray & Myerscough 1991; Murray 1993; Nagorcka & Mooney 1992). Although the equations for each model differ, they all share the basic requirement of the original model; that is, ‘waves’ are made from the interactions of two putative chemical substances which we refer to here as the ‘activator’ and the ‘inhibitor’ (Meinhardt 1982).
Key Terms
- Development Biology
- Mathematical Biology
- Biomathematics
- Morphogenesis
- Phyllotaxis
- Evolutionary Biology
- Nonlinear dynamical systems
- Cellular Automata
- Fractals
- Iterated Systems
- L-Systems
- Pattern Formation
- IFS (Iterated Functions Set)
- Theoretical Biology
- diffusion–reaction (DR) model
- Systems Biology
- Code Biology
- Computational Biology
- Algorithmic Biology
- Complex Systems
- Turing Patterns
Key People:
- D’Arcy Wentworth Thompson
- Aristid Lindenmayer
- Alan Turing
- Hans Meinhardt
- Philip Ball
- Przemyslaw Prusinkiewicz
- Murray JD
- Stephen Wolfram
Key Sources of Research:
On Growth and Form
Thompson D’Arcy W.
(1952)
The Algorithmic Beauty of Plants
Prusinkiewicz, Przemyslaw, Lindenmayer, Aristid
The Algorithmic Beauty of Seashells
Meinhardt H, Prusinkiewicz P, Fowler D
(2003)
(Springer, New York), 3rd Ed.
The Algorithmic Beauty of Seaweeds, Sponges and Corals
Kaandorp, Jaap A., Kübler, Janet E.
Mathematical Biology
Murray JD
(2003)
Models of biological pattern formation
Meinhardt H
(1982)
Pattern formation by coupled oscillations: The pigmentation patterns on the shells of molluscs
Hans Meinhardt, Martin Klingler
The Self-Made Tapestry Pattern formation in nature
Philip Ball
1999
Models of biological pattern formation in space and time
Hans Meinhardt
2014
Cellular Automata, PDEs, and Pattern Formation
The Computational Beauty of Nature: Computer Explorations of Fractals, Chaos, Complex Systems, and Adaptation
By Gary William Flake
The Curves of Life
Cook, T
1979
Dover Publications, Inc. New York.
Reaction-Diffusion Model as a Framework for Understanding Biological Pattern Formation
Shigeru Kondo1* and Takashi Miura
2010
Click to access kondomiura10science.pdf
The Hegemony of Molecular Biology
PHILIP KITCHER
Click to access kitcher99-hegemony.pdf
Modeling seashells
Deborah R. Fowlery, Hans Meinhardtz and Przemyslaw Prusinkiewicz
Click to access shells.sig92.pdf
The neural origins of shell structure and pattern in aquatic mollusks
Alistair Boettigera, Bard Ermentroutb, and George Oster
2009
Mechanical basis of morphogenesis and convergent evolution of spiny seashells
Régis Chirata, Derek E. Moultonb,1, and Alain Goriely
2013
The Geometry and Pigmentation of Seashells
S Coombes
2009
Click to access Seashells09.pdf
PATTERNS IN NATURE
Richie Khandelwal
Sahil Sahni
Forging patterns and making waves from biology to geology: a commentary on Turing (1952) ‘The chemical basis of morphogenesis’
Philip Ball
Click to access f989a13264a455ec2898ed361b1c435b5f0c.pdf
Mollusc Shell Pigmentation: Cellular Automaton Simulations and Evidence for Undecidability
INGO KUSCH AND MARIO MARKUS
1995
Click to access KuschMarkus1996.pdf
Pattern Formation in Reaction-Diffusion Systems
Masayasu Mimura
Click to access 7adbe7e696d4ba9ad3a89fed4ba15549a091.pdf
The Natural 3D Spiral
Gur Harary and Ayellet Tal
Click to access 11-HararyTal.pdf
A Model for Pattern Formation on the Shells of Molluscs
The reaction-diffusion system: a mechanism for autonomous pattern formation in the animal skin
Shigeru Kondo
Click to access The%20reaction-diffusion%20system_%20a%20mechanism%20for%20autonomous.pdf
Mechanical growth and morphogenesis of seashells
Derek E. Moulton, Alain Goriely and R ́egis Chirat
Scaling of morphogenetic patterns in continuous and discrete models
Click to access RasolonjanaharyMan_Sep2013_17293.pdf
On the Dynamics of a Forced Reaction-Diffusion Model for Biological Pattern Formation
A A Tsonis, JB Elsner, P A Tsonis
Impact of Turing’s Work
Maini
The possible role of reaction–diffusion in leaf shape
Nigel R. Franks1* and Nicholas F. Britton
Pattern regulation in the stripe of zebrafish suggests an underlying dynamic and autonomous mechanism
Motoomi Yamaguchi*†, Eiichi Yoshimoto‡, and Shigeru Kondo
Click to access yamaguchi2007.pdf
Turing Patterns
P Ball
Web Resource for Algorithmic Botony
http://algorithmicbotany.org/papers/
FRACTAL GEOMETRY AND SUPERFORMULA TO MODEL NATURAL SHAPES
Nicoletta Sala
2013
Click to access ijrras_16_4_09.pdf
The Geometry of Seashells
Dr S Coombes
SEASHELLS: THE PLAINNESS AND BEAUTY OF THEIR MATHEMATICAL DESCRIPTION
JORGE PICADO
Models for the morphogenesis of the molluscan shell
Click to access molluscanshell.pdf
Modeling Seashell Morphology
Exploring Complex Forms in Nature Through Mathematical Modeling: A Case on Turritella Terebra
Click to access ecaade2009_164.content.pdf
The Neural Origins of Sea Shell Patterns
Biological Pattern Formation : from Basic Mechanisms to Complex Structures
A. J. Kochy and H. Meinhardt
Form-Optimizing in Biological Structures The Morphology of Seashells
EDGAR STACH University of Tennessee
A Theory of Biological Pattern Formation
A. Gierer and H. Meinhardt
1972
Click to access gierer_meinhardt.pdf
Cellular Automata as Models of Complexity
Stephen Wolfram,
Nature 311 (5985): 419–424, 1984
Click to access 006_Wolfram1984.pdf
Website on Oliva Porphyria
http://oliva.porphyria.free.fr/menu%20GB.html
Evolution of patterns on Conus shells
Zhenqiang Gonga, Nichilos J. Matzkeb, Bard Ermentroutc, Dawn Songa, Jann E. Vendettib, Montgomery Slatkinb, and George Oster
Click to access 2012%20Evolution%20of%20patterns%20on%20Conus%20shells%20_E234.full.pdf
Theoretical aspects of pattern formation and neuronal development
http://www.eb.tuebingen.mpg.de/de/forschung/emeriti/hans-meinhardt/home.html
20+ Photos Of Geometrical Plants For Symmetry Lovers
http://www.boredpanda.com/geometry-symmetry-plants-nature/
Computational models of plant development and form
Przemyslaw Prusinkiewicz and Adam Runions
Click to access tansley.np2012.pdf
Periodic pattern formation in reaction–diffusion systems: An introduction for numerical simulation
Takashi Miura* and Philip K. Maini
Dynamics of Complex Systems
Yaneer Bar-yam

11 thoughts on “Shapes and Patterns in Nature”